Plants have astounding genetic diversity, with genomes that range from about 38 million base pairs of genes (Mb) to over 87,000 Mb. For comparison, the human genome consists of about 3,000 Mb. This remarkable genetic toolkit is a product of 400 million years of evolution by organisms that are rooted in place amidst ever-changing ecosystems and climate pressures.
While animals can move in search of water, food, safety, and mates, plants must adapt in place. Plants have even evolved to control the behavior of mobile species around them, employing strategies such as phytochemicals to evade predation and offering fruit and nectar in exchange for pollination, seed dispersal, and protection. These insect, animal, and human interactions shaped plant evolution, including our ~12,000 years of agriculture practices.
Before the 1800s, plant breeding meant saving seeds with preferred, visible traits from the individuals that survived a growing season. A breakthrough came in the 19th century with Gregor Mendel, an Augustinian monk whose meticulous experiments with pea plants revealed predictable patterns of inheritance. By crossbreeding plants with different traits, such as flower color or seed shape, he discovered the basic principles of heredity. Mendel is considered the father of modern genetics, and his discoveries laid the foundation for scientifically guided plant breeding.

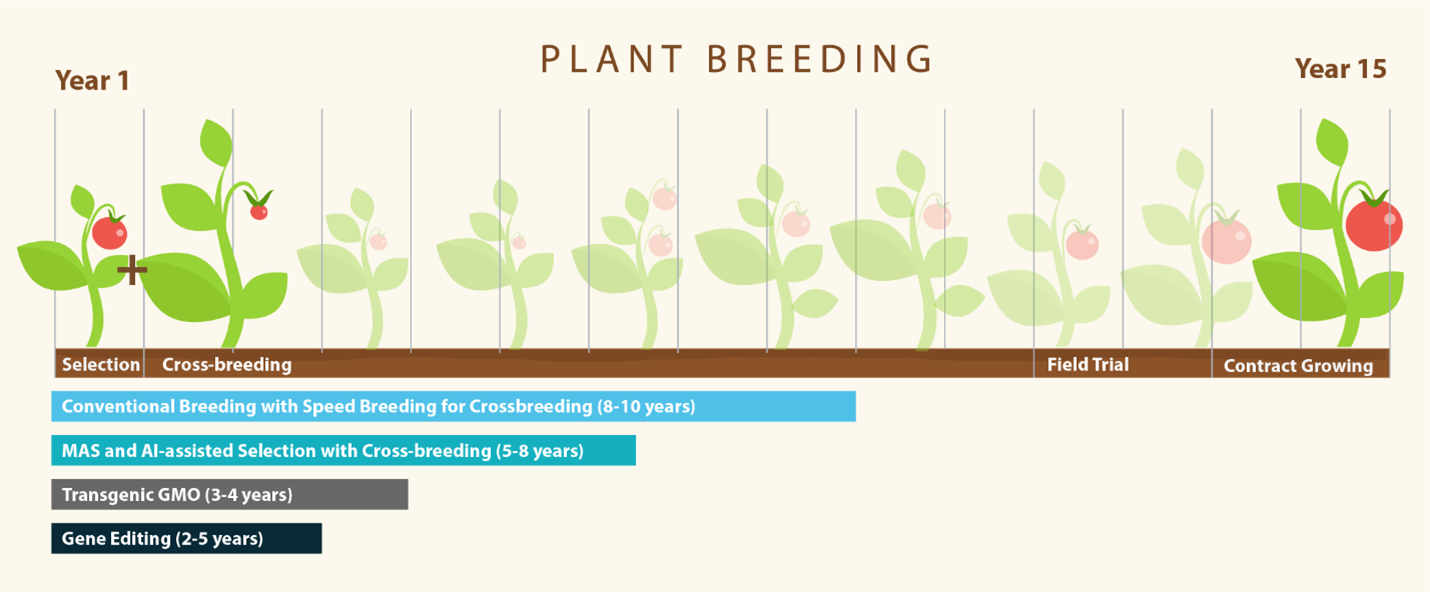
Despite today's more robust scientific knowledge, conventional seed breeding can require over a decade or more to develop desired traits, field-test the variety, and then grow it for market. Additionally, many varieties developed never reach the market. A key concern with conventional breeding is the method cannot develop resilient plants at the pace of our changing climate.
Today, seed breeders apply technological advancements to inform and accelerate breeding and even directly alter plant genomes. This post provides background on the technologies in use and their impact on regulatory policy and our food system.
To Breed with Speed
Seed breeders have developed creative approaches to reduce the timeline, such as shuttle breeding, where plants are grown in two separate locations with different climates, allowing for multiple generations in a single year. Another technique, speed breeding, utilizes extended light periods and controlled growing conditions to halve the time required to develop plant traits.
Technology-Powered Conventional Breeding
Seed selection and breeding are on the cusp of another seismic shift: readily available technology to map genomes and identify beneficial traits (such as marker-assisted selection or MAS) and machine learning or AI to model breeding outcomes. Mapping of the human genome revolutionized how genetic diseases can be treated, resulting in many beneficial medical advances. The same technology will be equally powerful to inform seed breeding (Krishna et al., 2023).
AI utilizes the massive data from genome mapping paired with data on the phenotypic expression of a plant variety in various soil and climate conditions to predict the results of crossbreeding different plant varieties. The technology can better target seed breeding, increasing the likelihood of developing a variety with the desired traits. Such technology can be paired with conventional or speed breeding to bring new varieties to market quicker as more genomic and phenotypic data become available.
Genetically Modified Organisms vs. Gene Editing
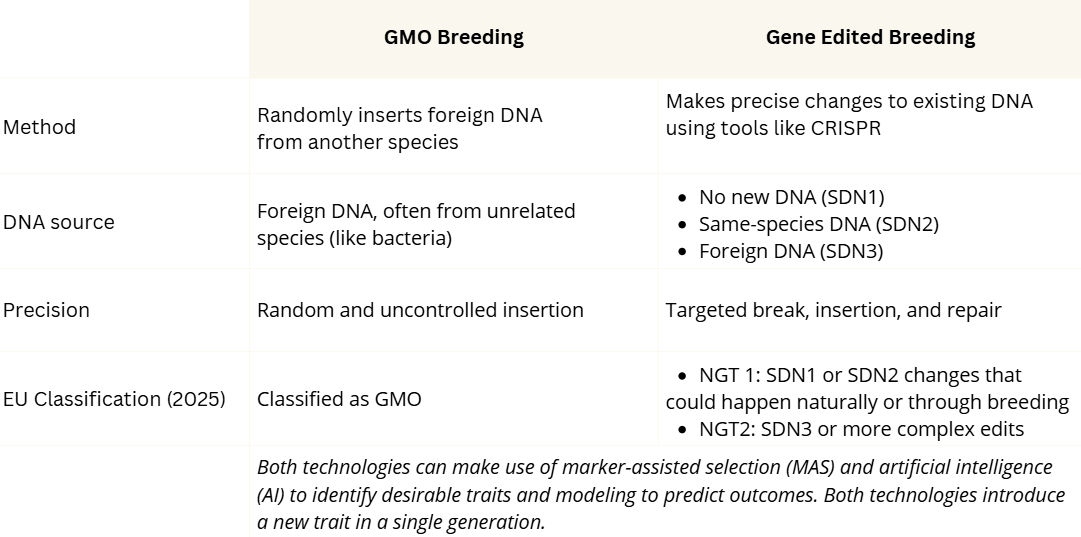
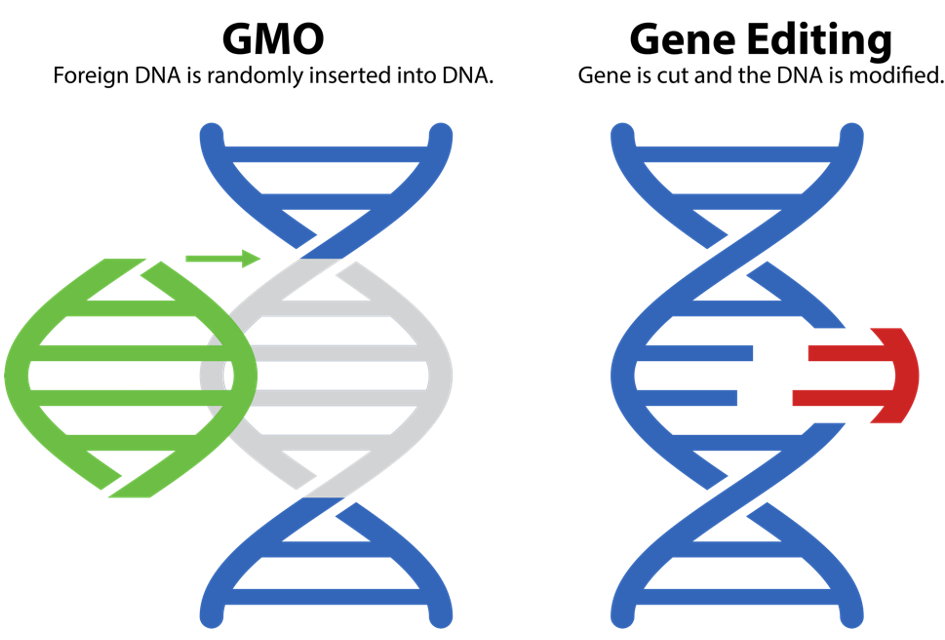
Today, scientists use powerful tools like artificial intelligence (AI), MAS, and genetic technologies to develop crops even faster. These tools allow researchers to directly change or add genetic material in plants, sometimes creating new traits in a single generation. One approach is genetic modification, used to make genetically modified organisms (GMOs), and another one is gene editing. While both involve changing the plant’s DNA, these technologies work in different ways.
Genetically Modified Organisms
The first GMO crop, a tomato released in 1992, was designed to ripen more slowly and stay fresh longer. It was made by inserting a gene that delayed softening. GMO crops are transgenic, meaning they carry foreign DNA, often from unrelated species.
For example, Bt corn and cotton were engineered using a gene from Bacillus thuringiensis, a soil bacterium that naturally produces an insecticide. In this case, the desirable trait (pest resistance) was identified in this bacterium, and the gene responsible for it was isolated and inserted into the plant’s genome. This allows the plant to defend itself against certain insects.
However, because the foreign DNA is inserted randomly, the exact location and effect of the genetic change can be unpredictable. This transgenic process has raised concerns about potential unintended effects that might not occur through natural breeding (Ahmad et al., 2023).
Gene Editing
Gene editing, on the other hand, is a method that allows scientists to make very precise changes to a plant’s DNA. A common tool for gene editing is called CRISPR, which works like tiny scissors that can cut a specific part of the DNA. After the cut is made, the plant’s natural repair system fixes it, but how it is fixed can vary.
- If the plant repairs the cut on its own without adding any new DNA, it’s called an SDN1 edit (Site Directed Nuclease).
- If a small piece of helpful DNA (from the same plant species) is added during the repair, it’s called an SDN2 edit.
- If DNA from another species is added (foreign DNA), it’s called an SDN3 edit.
So, SDN1 and SDN2 edits do not involve foreign DNA, while SDN3 does, which is similar to how GMOs are made.
Concerns with gene editing are similar to those with GMOs:
- Off-target effects of gene editing are not fully understood.
- Patents by biotech companies may further drive ownership and control of seed varieties (seed sovereignty and farmer rights).
- Unintended consequences of transgenic and modified genetics on landrace and wild plant genomes (Idris et al., 2023).
Regulatory Issues
The differences between SDN1 or SDN2 and SDN3 genetic editing can determine how gene edited crops are regulated, depending on the country (view a map with links to regulatory differences). In the US, where GMO crops are required to be labeled, SDN1 and SDN2 crops are not considered GMO crops as the genetic changes could have occurred through breeding or natural mutations (because no foreign DNA is introduced). These crops and seeds will not have to be labelled as genetically modified (Ahmad et al., 2023; Genetic Literacy Project, 2024).
The EU, however, is still determining the regulatory requirement for New Genomic Technologies (NGTs) like gene editing. The pending EU regulations are based on NGT1 and NGT2 categories.
- NGT1 includes modifications that could have occurred naturally or through conventional breeding. NGT1 crops would not have to be labeled as GMO, however seeds produced with these methods would require labeling.
- NGT2 includes all other genetic modifications, treating these as GMOs for regulatory purposes (European Council, 2025).
What Regulatory Differences Mean for Organics
The US regulatory approach will complicate traceability for the organic sector, which prohibits GMO and gene edited genetic modifications. For example, organic producers can currently use conventional seed for production if an organic variety is not available. Without labeling or traceability, it would be difficult to determine if the seed is not genetically edited.
The differences in labeling and traceability policies for gene edited crops across countries will further complicate preserving organic integrity for imports and exports. There is an urgent need for the sector to expand organic seed selections and improve traceability before gene edited technology becomes a more standard practice.
A Blast from the Past: Mutant Plants from Outer Space
“Mutant” plants sounds like a sci-fi movie title. However, scientists have been subjecting plants to radiation and chemicals to induce genetic mutations for a century, starting with radiating seeds in the early 1900s.
The extremes of space – high energy solar, cosmic radiation, and low gravity – have been the most effective means to induce significant mutations (Mohanta et al., 2021). China has developed over 200 seed varieties over the past 30 years using space mutagenesis (Liu et al.,2007). There are over 3,400 seed varieties currently listed in the Mutant Variety Database, maintained by the International Atomic Energy Agency (NOSB, 2023). Scientists are currently considering how the process can be used to develop seed varieties that are resilient to climate extremes (Orf, 2023).
Unlike GMO and gene editing approaches, induced mutagenesis (IM) plants still require crossing and breeding cultivars for several generations. While mutations are induced, rather than occurring naturally, no transgenic material is introduced.
The US National Organic Standards Board (NOSB) is currently considering if induced mutagenesis should be clearly defined as an excluded method, along with other plant breeding technologies (NOSB, 2023). The EU, US, and other countries currently do not recognize seed varieties produced by induced mutagenesis as genetically engineered.
The fruits of IM technology (literally) can be found in the produce section, including the much-loved ruby red grapefruit and Japanese pears. Yet few of us were aware of how these crops were developed, in part due to a lack of labeling requirements and policy.
Gene editing technology is more powerful and faster than IM, given the direct alteration of the genome. Gene edited crops do not have the years of crossbreeding. However, the slower-to-market timeline with IM provides a window to understand the effect of the mutations.
As the power and prevalence of genetic modification increases, it’s more important than ever to understand what these technologies are, how they are being applied to our food system, and how they might impact the ecosystems around us. As the saying goes, with great power comes great responsibility. And hopefully, careful thought and transparency.
Glossary
CRISPR: A genetic engineering tool that uses a CRISPR sequence of DNA and the associated protein (Cas9 in plants) to edit the base pairs of a gene in plants or other organisms.
Crossbreeding: The process of breeding two different varieties or species to create hybrids with specific traits.
Field trial: A test of a new variety outside the laboratory with requirements on location, plot size, growing techniques.
Gene editing: Type of genetic engineering in which DNA is deleted, inserted, modified or replaced in an organism’s genome.
Gene mapping: Determining the locations of genes on a chromosome.
Genetically modified organism (GMO): An organism produced through genetic modification, usually transgenic.
Genotype: The genetic identity of an individual.
Hybrid: The offspring of any cross between two organisms of different genotypes.
Induced Mutagenesis: Use of a chemical or physical mutation-causing agent to cause mutations in an organism's genetic code.
Marker assisted selection: Process where a genetic trait of interest is selected based on a marker that is linked the trait.
Mutation: Any heritable change in DNA structure or sequence.
Phenotype: The visible and/or measurable characteristics of an organism, the expression of the interaction of the genotype and the environment. A phenotype is not heritable.
Selective breeding: Deliberate crossing of organisms to produce desired characteristics in the next generation.
Transgenic: An organism that has had foreign genetic material introduced into its DNA.
References
Ahmad, A., Jamil, A., & Munawar, N. (2023). GMOs or non-GMOs? The CRISPR conundrum. Frontiers in Plant Science, 14. https://doi.org/10.3389/fpls.2023.1232938
European Council of the European Union. (2025,March 14). New genomic techniques: Council agrees negotiating mandate.Consilium.https://www.consilium.europa.eu/en/press/press-releases/2025/03/14/new-genomic-techniques-council-agrees-negotiating-mandate/
Farooq, M. A., Gao, S., Hassan, M. A., Huang, Z., Rasheed, A., Hearne, S., Prasanna, B., Li, X., & Li, H. (2024). Artificial intelligence in plant breeding. Trends in Genetics, 40(10), 891–908. https://doi.org/10.1016/j.tig.2024.07.001
Genetic Literacy Project. (2024, July 19). Global Gene Editing Regulation Tracker and Index. Global Gene Editing Regulation Tracker. https://crispr-gene-editing-regs-tracker.geneticliteracyproject.org/
Idris, S. H., Jalaluddin, N. S. M., &Chang, L. W. (2023). Ethical and legal implications of gene editing in plant breeding: a systematic literature review. Journal of Zhejiang University SCIENCE B, 24(12), 1093–1105. https://doi.org/10.1631/jzus.b2200601
Krishna, T. P. A., Veeramuthu, D.,Maharajan, T., & Soosaimanickam, M. (2023). The era of plant breeding: conventional breeding to genomics-assisted breeding for crop improvement. Current Genomics, 24(1), 24–35. https://doi.org/10.2174/1389202924666230517115912
Liu, Lu-xiang, Guo, Hui-jun, Zhao, Lin-shu,Gu, Jia-yu, Zhao, Shi-rong. (2007). ACHIEVEMENTS IN THE PAST TWENTY YEARS AND PERSPECTIVE OUTLOOK OF CROP SPACE BREEDING IN CHINA. (2007). Journal of Nuclear Agricultural Sciences. https://doi.org/10.11869/hnxb.2007.06.0589
Mohanta, T. K.,Mishra, A. K., Mohanta, Y. K., & Al-Harrasi, A. (2021). Space Breeding: the Next-Generation Crops. Frontiers in Plant Science, 12. https://doi.org/10.3389/fpls.2021.771985
NOSB. (2023, September 08). Induced Mutagenesis. Technical Evaluation Report Compiled by OMRI for the USDA National Organic Program. https://www.ams.usda.gov/sites/default/files/media/Induced_Mutagenesis.pdf
Orf, D. (2023, March 8). Mutated space seeds could be the answer to feeding our warming world. Popular Mechanics. https://www.popularmechanics.com/space/a43239127/space-seeds-on-iss/
Schwarzacher, T. (2011). The plant genome: an evolutionary view on structure and function. Organisation of the plant genome in chromosomes 1. https://www.le.ac.uk/biology/phh4/openpubs/hh_schwarzacher_chromosomes_plantjrevised.pdf
Tilman, D., & Lehman, C. (2001). Human-caused environmental change: Impacts on plant diversity and evolution. Proceedings of the National Academy of Sciences, 98(10), 5433–5440. https://doi.org/10.1073/pnas.091093198



